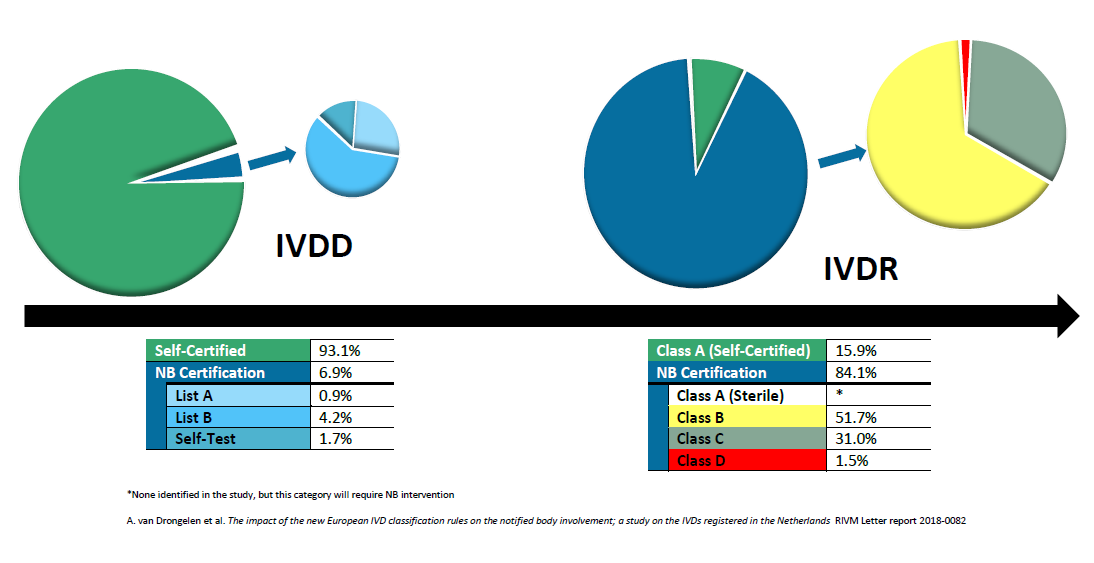
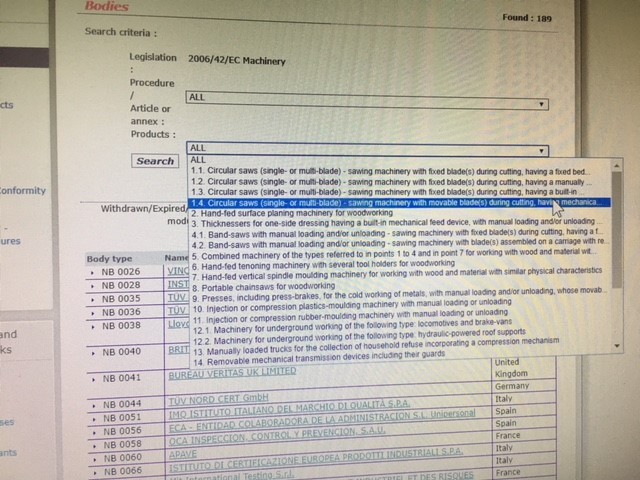
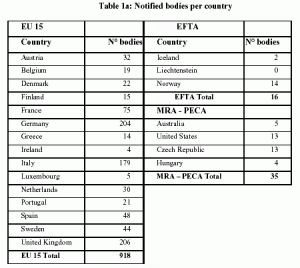
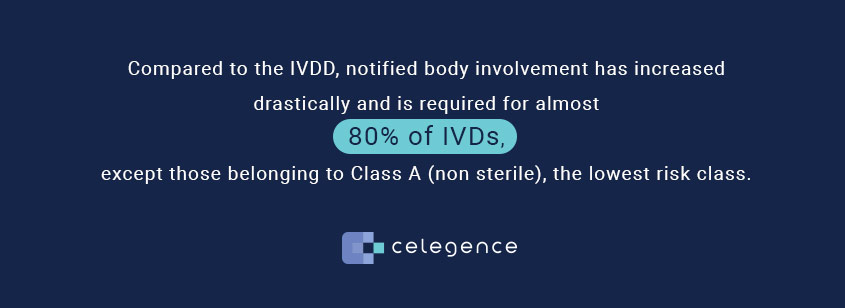
Auditing Organization (AO) versus Notified Body (NB) versus Registrar. What's the difference? – Oriel STAT A MATRIX – ELIQUENT Life Sciences Blog

List of Notified Bodies under IVDR (EU) 2017/746 on In-Vitro Diagnostic Medical Devices - Biotech Spain
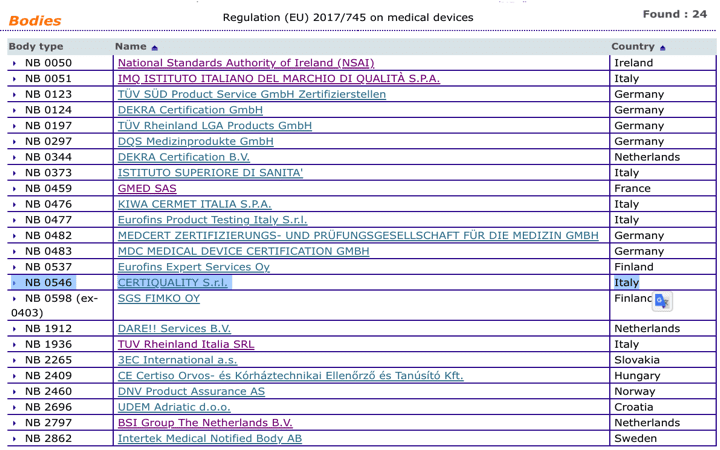
Organismos Notificados MDR (24): CERTIQUALITY (Italia) ON num. 0546 nuevo ON. Enhorabuena!!! | Red de Tecnologías Sanitarias y Productos Sanitarios
Availability and capacity of notified bodies to carry out conformity assessments for COVID-19 related medical devices and in vit