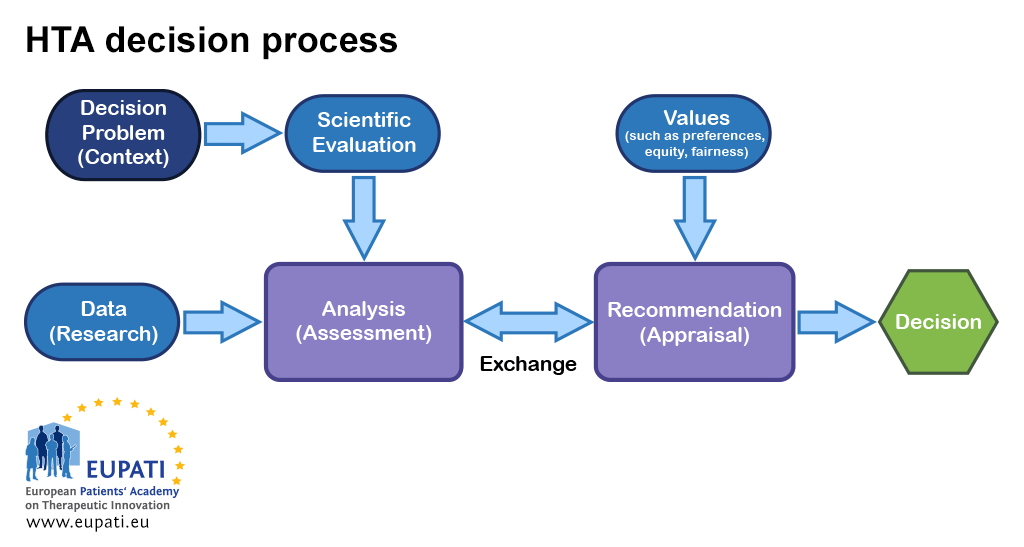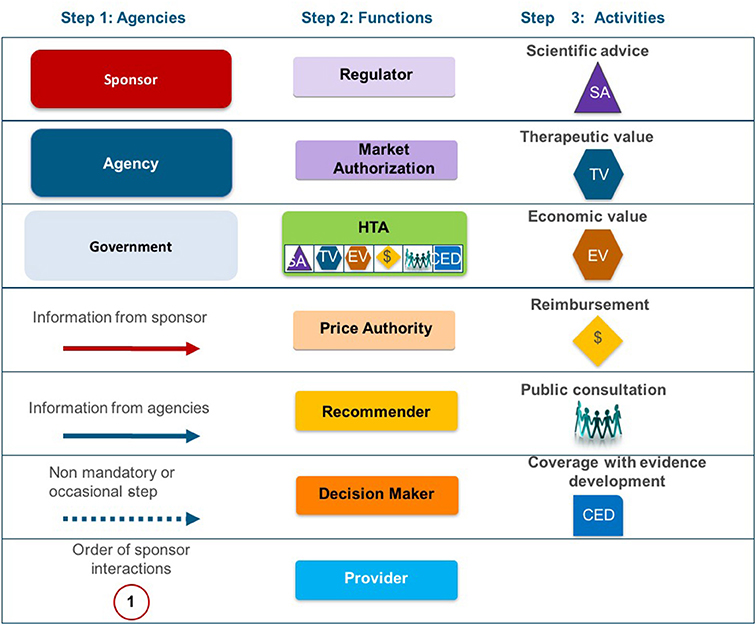
Frontiers | A Comparison of Reimbursement Recommendations by European HTA Agencies: Is There Opportunity for Further Alignment?
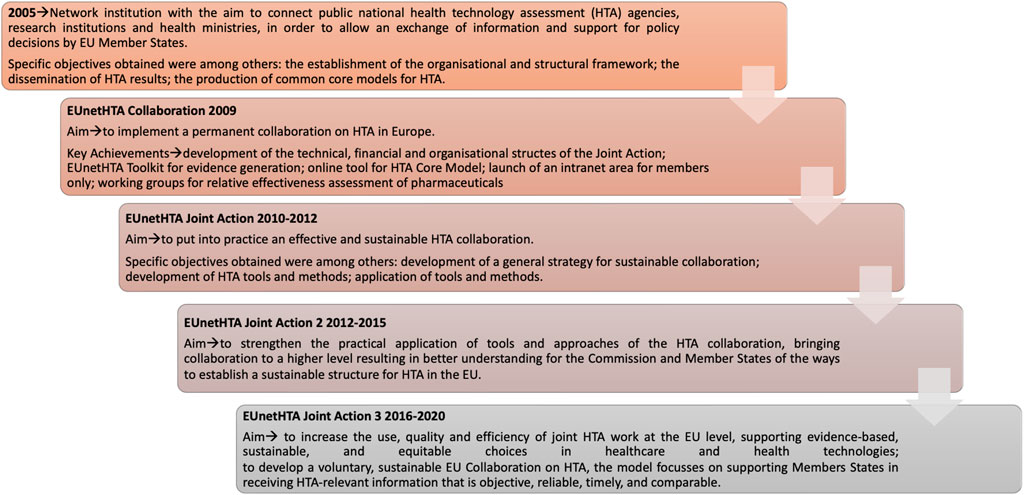
Frontiers | Towards a European harmonization of health technology assessment recommendations executive paper of European regulatory conference focused on the EU commission proposal to harmonize HTA
![PDF] Provisions and Special Considerations for Rare Diseases / Orphan Drugs by Health Technology Assessment (HTA) Bodies: Systematic Evaluation in 25 Countries | Semantic Scholar PDF] Provisions and Special Considerations for Rare Diseases / Orphan Drugs by Health Technology Assessment (HTA) Bodies: Systematic Evaluation in 25 Countries | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/14003250af4e607f52f1a5a0edead7f454917e31/1-Figure1-1.png)
PDF] Provisions and Special Considerations for Rare Diseases / Orphan Drugs by Health Technology Assessment (HTA) Bodies: Systematic Evaluation in 25 Countries | Semantic Scholar

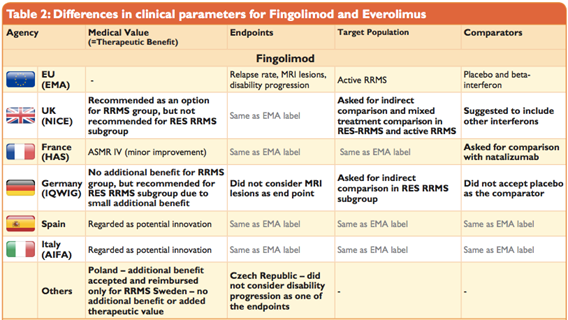
Full article: Differences in evidentiary requirements for oncology drug effectiveness assessments among six European health technology assessment bodies — can alignment be improved?

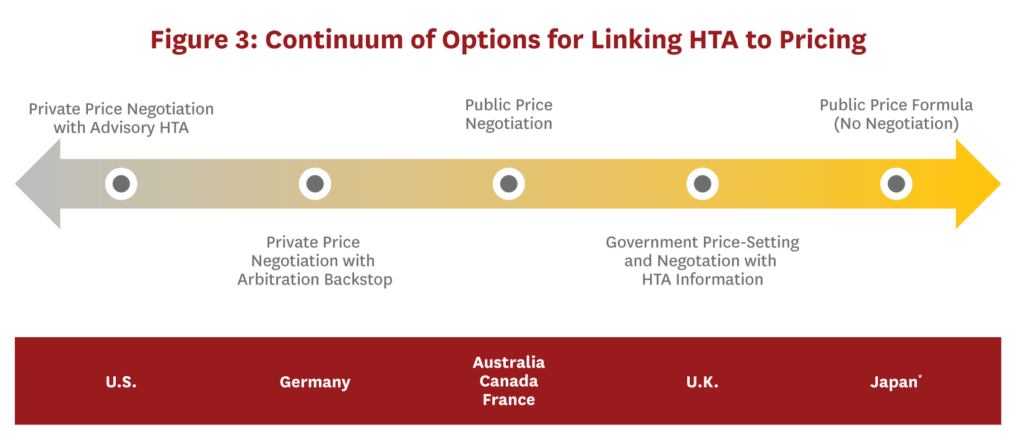
Similarities and Differences in Health Technology Assessment Systems and Implications for Coverage Decisions: Evidence from 32 Countries | PharmacoEconomics - Open
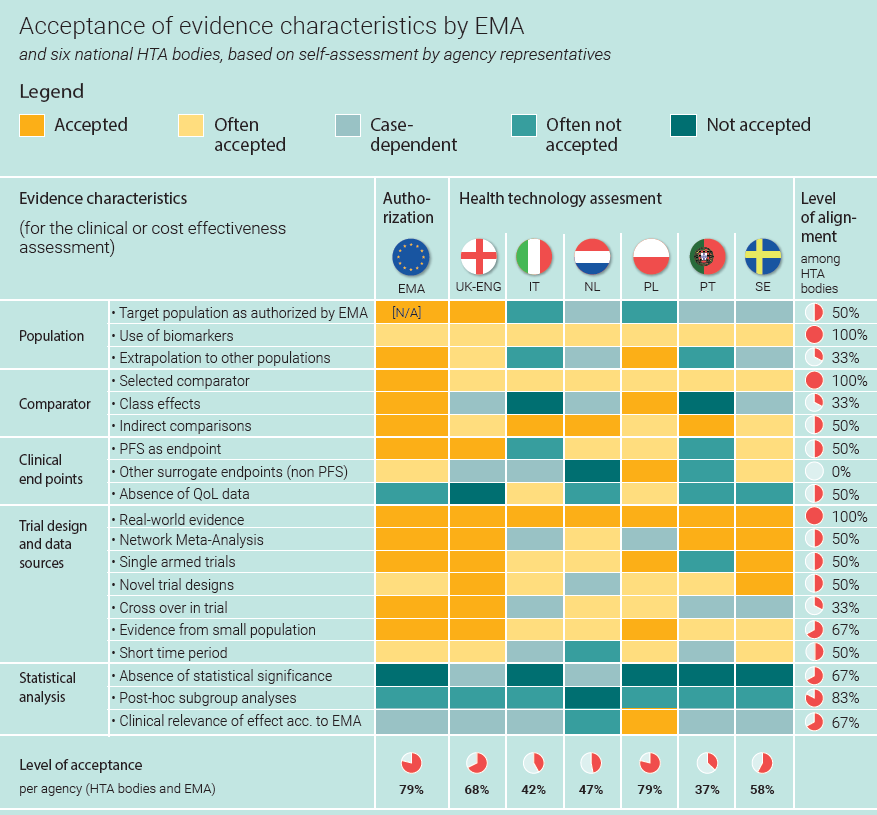
Europe's patchwork of evidence requirements is an important factor in delayed patient access - Consultancy in Healthcare and Life Sciences | Vintura Consultancy

Figure 5 from Health Technology Assessment (HTA) Case Studies: Factors Influencing Divergent HTA Reimbursement Recommendations in Australia, Canada, England, and Scotland. | Semantic Scholar

Known/published barriers to RWD/RWE uptake by HTA bodies and payers.... | Download Scientific Diagram

PDF) Weighing of Evidence by Health Technology Assessment Bodies: Retrospective Study of Reimbursement Recommendations for Conditionally Approved Drugs | Lourens T Bloem - Academia.edu
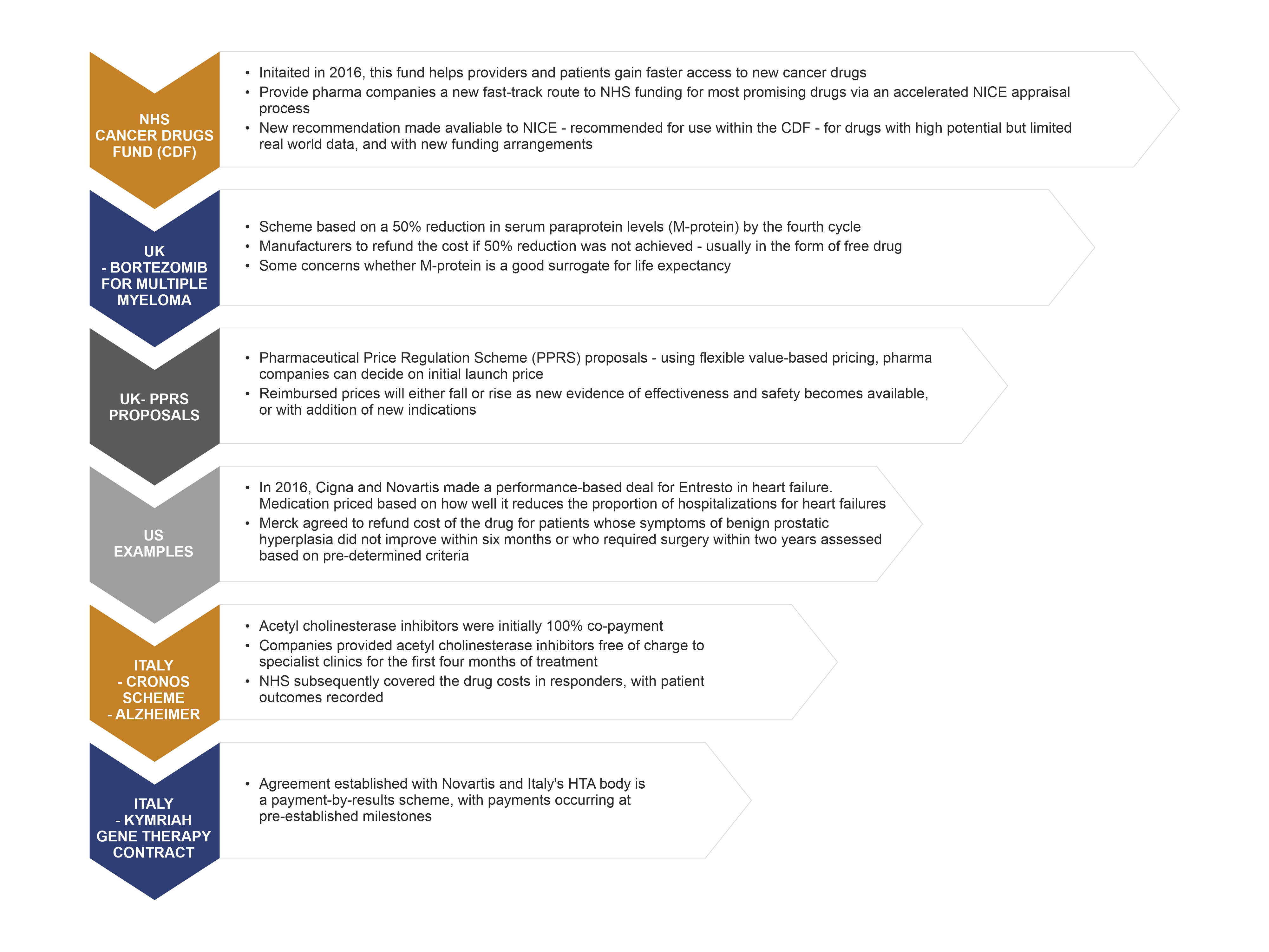
Early engagement with Health Technology Assessment authorities will accelerate product launch and improve chances for reimbursement

Health technology assessment of medical devices: a survey of non-European union agencies. - Abstract - Europe PMC